Bacteriophage:

History
Since ancient times, reports of river waters having the ability to cure infectious diseases have been documented, such as leprosy. In 1896, Ernest Hanbury Hankin reported that something in the waters of the Ganges and Yamuna rivers in India had marked antibacterial action against cholera and could pass through a very fine porcelain filter. In 1915, British bacteriologist Frederick Twort, superintendent of the Brown Institution of London, discovered a small agent that infected and killed bacteria. He believed the agent must be one of the following:
Independently, French-Canadian microbiologist Félix d'Hérelle, working at the Pasteur Institute in Paris, announced on 3 September 1917, that he had discovered "an invisible, antagonistic microbe of the dysentery bacillus". For d’Hérelle, there was no question as to the nature of his discovery: "In a flash I had understood: what caused my clear spots was in fact an invisible microbe ... a virus parasitic on bacteria."[6] D'Hérelle called the virus a bacteriophage or bacteria-eater (from the Greek phagein meaning to eat). He also recorded a dramatic account of a man suffering from dysentery who was restored to good health by the bacteriophages.[7]
In 1923, the Eliava Institute was opened in Tbilisi, Georgia, to research this new science and put it into practice.
In 1969, Max Delbrück, Alfred Hershey and Salvador Luria were awarded the Nobel Prize in Physiology and Medicine for their discoveries of the replication of viruses and their genetic structure.
In contrast, the lysogenic cycle does not result in immediate lysing of the host cell. Those phages able to undergo lysogeny are known as temperate phages. Their viral genome will integrate with host DNA and replicate along with it fairly harmlessly, or may even become established as a plasmid. The virus remains dormant until host conditions deteriorate, perhaps due to depletion of nutrients; then, the endogenous phages (known as prophages) become active. At this point they initiate the reproductive cycle, resulting in lysis of the host cell. As the lysogenic cycle allows the host cell to continue to survive and reproduce, the virus is reproduced in all of the cell’s offspring. An example of a bacteriophage known to follow the lysogenic cycle and the lytic cycle is the phage lambda of E. coli.[8]
Sometimes prophages may provide benefits to the host bacterium while they are dormant by adding new functions to the bacterial genome in a phenomenon called lysogenic conversion. An eminent example is the conversion of a harmless strain of Vibrio cholerae by a phage into a highly virulent one, which causes cholera.
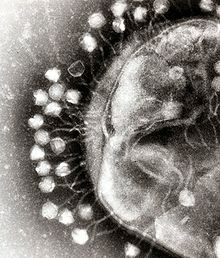
To enter a host cell, bacteriophages attach to specific receptors on the surface of bacteria, including lipopolysaccharides, teichoic acids, proteins, or even flagella. This specificity means a bacteriophage can infect only certain bacteria bearing receptors to which they can bind, which in turn determines the phage's host range. Host growth conditions also influence the ability of the phage to attach and invade them.[9] As phage virions do not move independently, they must rely on random encounters with the right receptors when in solution (blood, lymphatic circulation, irrigation, soil water, etc.).
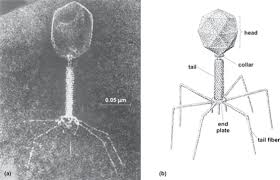
Myovirus bacteriophages use a hypodermic syringe-like motion to inject their genetic material into the cell. After making contact with the appropriate receptor, the tail fibers flex to bring the base plate closer to the surface of the cell; this is known as reversible binding. Once attached completely, irreversible binding is initiated and the tail contracts, possibly with the help of ATP present in the tail,[3] injecting genetic material through the bacterial membrane. Podoviruses lack an elongated tail sheath similar to that of a myovirus, so they instead use their small, tooth-like tail fibers to enzymatically degrade a portion of the cell membrane before inserting their genetic material.

Released virions are described as free, and, unless defective, are capable of infecting a new bacterium. Budding is associated with certain Mycoplasma phages. In contrast to virion release, phages displaying a lysogenic cycle do not kill the host but, rather, become long-term residents as prophage.
The first regulated clinical trial of efficacy in Western Europe (against ear infections caused by Pseudomonas aeruginosa) was reported in the journal Clinical Otolaryngology in August 2009.[14] Meanwhile, Western scientists are developing engineered viruses to overcome antibiotic resistance, and experimenting with tumor-suppressing agents.[15] One potential treatment currently under development is a phage designed to destroy MRSA.[16]
Bacteriophages have also been used in hydrological tracing and modelling in river systems, especially where surface water and groundwater interactions occur. The use of phages is preferred to the more conventional dye marker because they are significantly less absorbed when passing through ground waters and they are readily detected at very low concentrations.[18]
Government agencies in the West have for several years been looking to Georgia and the former Soviet Union for help with exploiting phages for counteracting bioweapons and toxins, such as anthrax and botulism.[22] Developments are continuing among research groups in the US. Other uses include spray application in horticulture for protecting plants and vegetable produce from decay and the spread of bacterial disease. Other applications for bacteriophages are as biocides for environmental surfaces, e.g., in hospitals, and as preventative treatments for catheters and medical devices prior to use in clinical settings. The technology for phages to be applied to dry surfaces, e.g., uniforms, curtains, or even sutures for surgery now exists. Clinical trials reported in the Lancet[14] show success in veterinary treatment of pet dogs with otitis.
Phage display is a different use of phages involving a library of phages with a variable peptide linked to a surface protein. Each phage's genome encodes the variant of the protein displayed on its surface (hence the name), providing a link between the peptide variant and its encoding gene. Variant phages from the library can be selected through their binding affinity to an immobilized molecule (e.g., botulism toxin) to neutralize it. The bound, selected phages can be multiplied by reinfecting a susceptible bacterial strain, thus allowing them to retrieve the peptides encoded in them for further study.[citation needed]
The SEPTIC bacterium sensing and identification method uses the ion emission and its dynamics during phage infection and offers high specificity and speed for detection.[citation needed]
Phage-ligand technology makes use of proteins, which are identified from bacteriophages, characterized and recombinantly expressed for various applications such as binding of bacteria and bacterial components (e.g. endotoxin) and lysis of bacteria.[23]
With its six legs, the bacteriophage attaches to the surface of the much larger bacteria Escherichia coli (E. coli).
Once attached, the bacteriophage injects DNA into the bacterium. The DNA instructs the bacterium to produce masses of new viruses.
So many are produced, that the E. coli bursts.
From Wikipedia, the free encyclopedia
A bacteriophage (from 'bacteria' and Greek φαγεῖν phagein "to devour") is any one of a number of viruses that infect bacteria. They do this by injecting genetic material, which they carry enclosed in an outer protein capsid. The genetic material can be ssRNA, dsRNA, ssDNA, or dsDNA ('ss-' or 'ds-' prefix denotes single-strand or double-strand) along with either circular or linear arrangement.
Bacteriophages are among the most common and diverse entities in the biosphere.[1] The term is commonly used in its shortened form, phage.
Phages are widely distributed in locations populated by bacterial hosts, such as soil or the intestines of animals. One of the densest natural sources for phages and other viruses is sea water, where up to 9×108 virions per milliliter have been found in microbial mats at the surface,[2] and up to 70% of marine bacteria may be infected by phages.[3] They have been used for over 90 years as an alternative to antibiotics in the former Soviet Union and Eastern Europe, as well as in France.[4] They are seen as a possible therapy against multi-drug-resistant strains of many bacteria.[5]
Classification
The dsDNA tailed phages, or Caudovirales, account for 95% of all the phages reported in the scientific literature, and possibly make up the majority of phages on the planet.[1] However, other phages occur abundantly in the biosphere, with different virions, genomes and lifestyles. Phages are classified by the International Committee on Taxonomy of Viruses (ICTV) according to morphology and nucleic acid.
Nineteen families are currently recognised that infect bacteria and archaea. Of these, only two families have RNA genomes and only five families are enveloped. Of the viral families with DNA genomes, only two have single-stranded genomes. Eight of the viral families with DNA genomes have circular genomes, while nine have linear genomes. Nine families infect bacteria only, nine infect archaea only, and one (Tectiviridae) infects both bacteria and archaea.
Bacteriophages are among the most common and diverse entities in the biosphere.[1] The term is commonly used in its shortened form, phage.
Phages are widely distributed in locations populated by bacterial hosts, such as soil or the intestines of animals. One of the densest natural sources for phages and other viruses is sea water, where up to 9×108 virions per milliliter have been found in microbial mats at the surface,[2] and up to 70% of marine bacteria may be infected by phages.[3] They have been used for over 90 years as an alternative to antibiotics in the former Soviet Union and Eastern Europe, as well as in France.[4] They are seen as a possible therapy against multi-drug-resistant strains of many bacteria.[5]
Classification
The dsDNA tailed phages, or Caudovirales, account for 95% of all the phages reported in the scientific literature, and possibly make up the majority of phages on the planet.[1] However, other phages occur abundantly in the biosphere, with different virions, genomes and lifestyles. Phages are classified by the International Committee on Taxonomy of Viruses (ICTV) according to morphology and nucleic acid.
Nineteen families are currently recognised that infect bacteria and archaea. Of these, only two families have RNA genomes and only five families are enveloped. Of the viral families with DNA genomes, only two have single-stranded genomes. Eight of the viral families with DNA genomes have circular genomes, while nine have linear genomes. Nine families infect bacteria only, nine infect archaea only, and one (Tectiviridae) infects both bacteria and archaea.
History
Since ancient times, reports of river waters having the ability to cure infectious diseases have been documented, such as leprosy. In 1896, Ernest Hanbury Hankin reported that something in the waters of the Ganges and Yamuna rivers in India had marked antibacterial action against cholera and could pass through a very fine porcelain filter. In 1915, British bacteriologist Frederick Twort, superintendent of the Brown Institution of London, discovered a small agent that infected and killed bacteria. He believed the agent must be one of the following:
- a stage in the life cycle of the bacteria;
- an enzyme produced by the bacteria themselves; or
- a virus that grew on and destroyed the bacteria.
Independently, French-Canadian microbiologist Félix d'Hérelle, working at the Pasteur Institute in Paris, announced on 3 September 1917, that he had discovered "an invisible, antagonistic microbe of the dysentery bacillus". For d’Hérelle, there was no question as to the nature of his discovery: "In a flash I had understood: what caused my clear spots was in fact an invisible microbe ... a virus parasitic on bacteria."[6] D'Hérelle called the virus a bacteriophage or bacteria-eater (from the Greek phagein meaning to eat). He also recorded a dramatic account of a man suffering from dysentery who was restored to good health by the bacteriophages.[7]
In 1923, the Eliava Institute was opened in Tbilisi, Georgia, to research this new science and put it into practice.
In 1969, Max Delbrück, Alfred Hershey and Salvador Luria were awarded the Nobel Prize in Physiology and Medicine for their discoveries of the replication of viruses and their genetic structure.
[edit] Replication
Bacteriophages may have a lytic cycle or a lysogenic cycle, and a few viruses are capable of carrying out both. With lytic phages such as the T4 phage, bacterial cells are broken open (lysed) and destroyed after immediate replication of the virion. As soon as the cell is destroyed, the phage progeny can find new hosts to infect. Lytic phages are more suitable for phage therapy. Some lytic phages undergo a phenomenon known as lysis inhibition, where completed phage progeny will not immediately lyse out of the cell if extracellular phage concentrations are high. This mechanism is not identical to that of temperate phage going dormant and is usually temporary.In contrast, the lysogenic cycle does not result in immediate lysing of the host cell. Those phages able to undergo lysogeny are known as temperate phages. Their viral genome will integrate with host DNA and replicate along with it fairly harmlessly, or may even become established as a plasmid. The virus remains dormant until host conditions deteriorate, perhaps due to depletion of nutrients; then, the endogenous phages (known as prophages) become active. At this point they initiate the reproductive cycle, resulting in lysis of the host cell. As the lysogenic cycle allows the host cell to continue to survive and reproduce, the virus is reproduced in all of the cell’s offspring. An example of a bacteriophage known to follow the lysogenic cycle and the lytic cycle is the phage lambda of E. coli.[8]
Sometimes prophages may provide benefits to the host bacterium while they are dormant by adding new functions to the bacterial genome in a phenomenon called lysogenic conversion. An eminent example is the conversion of a harmless strain of Vibrio cholerae by a phage into a highly virulent one, which causes cholera.
[edit] Attachment and penetration

In this electron micrograph of bacteriophages attached to a bacterial cell, the viruses are the size and shape of coliphage T1.
Myovirus bacteriophages use a hypodermic syringe-like motion to inject their genetic material into the cell. After making contact with the appropriate receptor, the tail fibers flex to bring the base plate closer to the surface of the cell; this is known as reversible binding. Once attached completely, irreversible binding is initiated and the tail contracts, possibly with the help of ATP present in the tail,[3] injecting genetic material through the bacterial membrane. Podoviruses lack an elongated tail sheath similar to that of a myovirus, so they instead use their small, tooth-like tail fibers to enzymatically degrade a portion of the cell membrane before inserting their genetic material.
[edit] Synthesis of proteins and nucleic acid
Within minutes, bacterial ribosomes start translating viral mRNA into protein. For RNA-based phages, RNA replicase is synthesized early in the process. Proteins modify the bacterial RNA polymerase so it preferentially transcribes viral mRNA. The host’s normal synthesis of proteins and nucleic acids is disrupted, and it is forced to manufacture viral products, instead. These products go on to become part of new virions within the cell, helper proteins that help assemble the new virions, or proteins involved in cell lysis. Walter Fiers (University of Ghent, Belgium) was the first to establish the complete nucleotide sequence of a gene (1972) and of the viral genome of bacteriophage MS2 (1976).[10][edit] Virion assembly
In the case of the T4 phage, the construction of new virus particles involves the assistance of helper proteins. The base plates are assembled first, with the tails being built upon them afterwards. The head capsids, constructed separately, will spontaneously assemble with the tails. The DNA is packed efficiently within the heads. The whole process takes about 15 minutes.[edit] Release of virions
Phages may be released via cell lysis, by extrusion, or, in a few cases, by budding. Lysis, by tailed phages, is achieved by an enzyme called endolysin, which attacks and breaks down the cell wall peptidoglycan. An altogether different phage type, the filamentous phages, make the host cell continually secrete new virus particles.Released virions are described as free, and, unless defective, are capable of infecting a new bacterium. Budding is associated with certain Mycoplasma phages. In contrast to virion release, phages displaying a lysogenic cycle do not kill the host but, rather, become long-term residents as prophage.
[edit] Genome structure
Bacteriophage genomes are especially mosaic: the genome of any one phage species appears to be composed of numerous individual modules. These modules may be found in other phage species in different arrangements. Mycobacteriophages - bacteriophages with mycobacterial hosts - have provided excellent examples of this mosaicism. In these mycobacteriophages, genetic assortment may be the result of repeated instances of site-specific recombination and illegitimate recombination (the result of phage genome acquisition of bacterial host genetic sequences).[11] It should be noted, however, that evolutionary mechanisms shaping the genomes of bacterial viruses vary between different families and depend on the type of the nucleic acid, characteristics of the virion structure, as well as the mode of the viral life cycle.[12][edit] Phage therapy
Main article: Phage therapy
Phages were discovered to be antibacterial agents and were used in the United States and Europe during the 1920s and 1930s for treating bacterial infections. They had widespread use, including treating soldiers in the Red Army. However, they were abandoned for general use in the West for several reasons:- Medical trials were carried out, but a basic lack of understanding of phages made these invalid.
- Phage therapy was seen as untrustworthy, because many of the trials were conducted on totally unrelated diseases such as allergies and viral infections.
- Antibiotics were discovered and marketed widely. They were easier to make, store and to prescribe.
- Russian research continued, but was published in Russian or Georgian, and was unavailable internationally for many years.
The first regulated clinical trial of efficacy in Western Europe (against ear infections caused by Pseudomonas aeruginosa) was reported in the journal Clinical Otolaryngology in August 2009.[14] Meanwhile, Western scientists are developing engineered viruses to overcome antibiotic resistance, and experimenting with tumor-suppressing agents.[15] One potential treatment currently under development is a phage designed to destroy MRSA.[16]
[edit] In the environment
Main article: Marine bacteriophage
Metagenomics has allowed the in-water detection of bacteriophages that was not possible previously. These investigations revealed phages are much more abundant in the water column of both freshwater and marine habitats than previously thought,[17] and they can cause significant mortality of bacterioplankton. Methods in phage community ecology have been developed to assess phage-induced mortality of bacterioplankton and its role for food web process and biogeochemical cycle to genetically fingerprint phage communities or populations and estimate viral biodiversity by metagenomics. The lysis of bacteria by phages releases organic carbon that was previously particulate (cells) into dissolved forms, which makes the carbon more available to other organisms. Phages are not only the most abundant biological entities but also probably also the most diverse ones. The majority of the sequence data obtained from phage communities have no equivalents in databases. These data and other detailed analyses indicate phage-specific genes and ecological traits are much more frequent than previously thought. To reveal the meaning of this genetic and ecological versatility, studies have to be performed with communities and at spatiotemporal scales relevant for microorganisms.[1]Bacteriophages have also been used in hydrological tracing and modelling in river systems, especially where surface water and groundwater interactions occur. The use of phages is preferred to the more conventional dye marker because they are significantly less absorbed when passing through ground waters and they are readily detected at very low concentrations.[18]
[edit] Role in food fermentation
A broad number of food products, commodity chemicals, and biotechnology products are manufactured industrially by large-scale bacterial fermentation of various organic substrates. Because enormous amounts of bacteria are being cultivated each day in large fermentation vats, the risk of bacteriophage contamination could rapidly bring fermentation to a halt. The resulting economic setback is a serious threat in these industries. The relationship between bacteriophages and their bacterial hosts is very important in the context of the food fermentation industry. Sources of phage contamination, measures to control their propagation and dissemination, and biotechnological defense strategies developed to restrain phages are of interest. The dairy fermentation industry has openly acknowledged the problem of phages and has been working with academia and starter culture companies to develop defense strategies and systems to curtail the propagation and evolution of phages for decades.[1][edit] Other areas of use
Since 2006, the United States Food and Drug Administration (FDA) and USDA have approved several bacteriophage products. Intralytix introduced LMP-102, also called ListShield as a food additive to target and kill Listeria monocytogenes. LMP-102 (ListShield by Intralytix) was approved for treating ready-to-eat (RTE) poultry and meat products. In that same year, the FDA approved LISTEX by MICREOS (formerly EBI) using bacteriophages on cheese to kill the L. monocytogenes bacteria, giving them generally recognized as safe (GRAS) status.[19] In July 2007, the same bacteriophage were approved for use on all food products.[20] In 2011 USDA confirmed that LISTEX is a clean label processing-aid and is included in USDA.[21] Research in the field of food safety is continuing to see if lytic phages are a viable option to control other food-borne pathogens in various food products.Government agencies in the West have for several years been looking to Georgia and the former Soviet Union for help with exploiting phages for counteracting bioweapons and toxins, such as anthrax and botulism.[22] Developments are continuing among research groups in the US. Other uses include spray application in horticulture for protecting plants and vegetable produce from decay and the spread of bacterial disease. Other applications for bacteriophages are as biocides for environmental surfaces, e.g., in hospitals, and as preventative treatments for catheters and medical devices prior to use in clinical settings. The technology for phages to be applied to dry surfaces, e.g., uniforms, curtains, or even sutures for surgery now exists. Clinical trials reported in the Lancet[14] show success in veterinary treatment of pet dogs with otitis.
Phage display is a different use of phages involving a library of phages with a variable peptide linked to a surface protein. Each phage's genome encodes the variant of the protein displayed on its surface (hence the name), providing a link between the peptide variant and its encoding gene. Variant phages from the library can be selected through their binding affinity to an immobilized molecule (e.g., botulism toxin) to neutralize it. The bound, selected phages can be multiplied by reinfecting a susceptible bacterial strain, thus allowing them to retrieve the peptides encoded in them for further study.[citation needed]
The SEPTIC bacterium sensing and identification method uses the ion emission and its dynamics during phage infection and offers high specificity and speed for detection.[citation needed]
Phage-ligand technology makes use of proteins, which are identified from bacteriophages, characterized and recombinantly expressed for various applications such as binding of bacteria and bacterial components (e.g. endotoxin) and lysis of bacteria.[23]
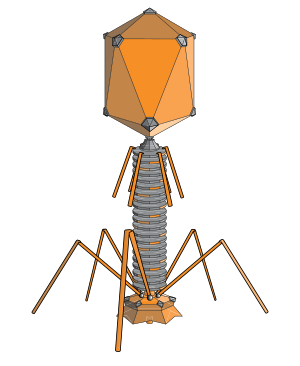
The Virus that Infect Bacteria
T4 bacteriophage is a virus that looks like an alien landing pod.With its six legs, the bacteriophage attaches to the surface of the much larger bacteria Escherichia coli (E. coli).
Once attached, the bacteriophage injects DNA into the bacterium. The DNA instructs the bacterium to produce masses of new viruses.
So many are produced, that the E. coli bursts.
Viruses and Bacteria
It's easy to mix these up since compared to us, both are VERY SMALL.
But bacteria, given the proper nutrients, can grow and reproduce on their own. Viruses cannot "live" or reproduce without getting inside some living cell, whether it's a plant, animal, or bacteria.
And compared to viruses, bacteria are huge
Now you decided........








No comments:
Post a Comment